Guides
Alkalinity: Sodium Bicarbonate vs Sodium Carbonate – Choosing the Right Supplement
Alkalinity plays a critical role in reef aquariums, directly influencing coral health, pH stability, and overall system balance. For many reefers, the question often comes down to choosing between sodium bicarbonate and sodium carbonate as their preferred alkalinity supplement. Understanding how each one works and when to use it can make the difference between thriving coral growth and slow, inconsistent calcification.
Let’s explore the theory behind alkalinity, the chemistry of carbonate and bicarbonate, and how to pick the right supplement for your system.

Understanding Alkalinity and Coral Calcification
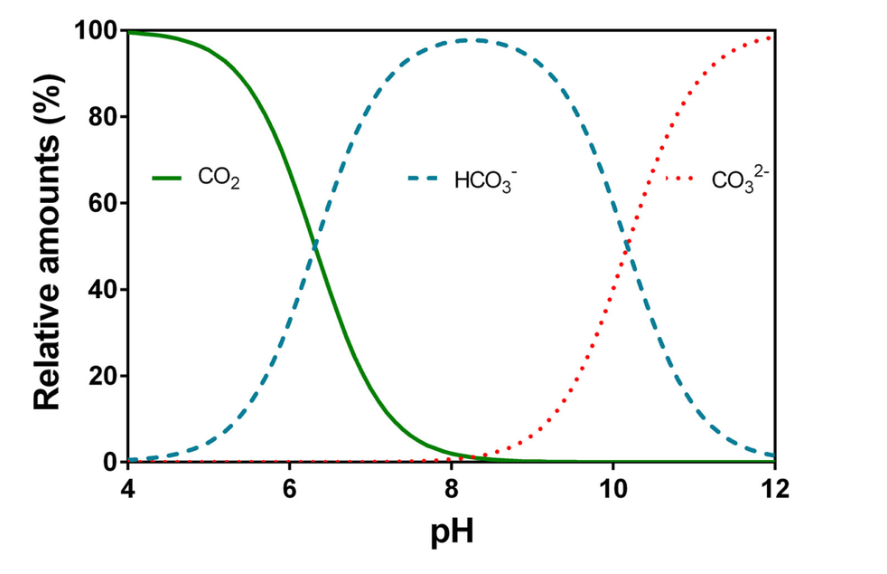
Corals consume both carbonate (CO₃²⁻) and bicarbonate (HCO₃⁻) ions to build their calcium carbonate skeletons. Although they can utilize both, they prefer carbonate ions because corals don’t need to spend extra energy removing a hydrogen atom. This process is central to coral growth and is affected by pH, which determines the balance between carbonate and bicarbonate in seawater.
In simple terms:
- At lower pH (acidic): More CO₂ and bicarbonate (HCO₃⁻)
- At higher pH (basic): More carbonate (CO₃²⁻)
Here’s how the balance shifts under typical reef conditions:
- pH 8.0 → ~90% bicarbonate and 10% carbonate
- pH 8.3 → ~85% bicarbonate and 15% carbonate
- pH 8.6 → ~80% bicarbonate and 20% carbonate
Since carbonate is the preferred form for coral skeleton building, maintaining ideal alkalinity and pH is essential for optimal coral growth.
Carbonate vs Bicarbonate balance at different pH levels (blue is the bicarbonate, red is the carbonate)
Why “Perfect Ocean Ratios” Are a Myth
Some supplement manufacturers claim their alkalinity products replicate the “exact ocean water ratio” of bicarbonate and carbonate. In reality, this claim doesn’t hold up. The ratio between the two isn’t fixed by what you dose—it’s determined by pH, temperature, and salinity.
Dosing carbonate or bicarbonate can change total alkalinity, but not the internal ratio between these ions. What truly controls that balance is how your tank’s chemistry stabilizes around its pH level.
Therefore, reefers should focus less on “perfect ocean ratios” and more on understanding their aquarium’s current pH, total alkalinity, and how each supplement affects those values.
How to Shift the Carbonate-Bicarbonate Balance
Even though you can’t directly control the ratio by dosing, you can influence it indirectly through pH management.
To shift toward more carbonate (CO₃²⁻) for example you can:
- Raise pH by improving gas exchange
- Dose kalkwasser (especially at night)
- Use a CO₂ scrubber
- Run a lighted refugium overnight
By understanding these dynamics, reefers can tailor alkalinity supplementation to their system’s unique chemistry rather than relying on one-size-fits-all solutions.
Sodium Bicarbonate: Gentle Alkalinity Boost Without Raising pH
Sodium bicarbonate (NaHCO₃) increases alkalinity with minimal effect on pH. This makes it ideal for tanks where the pH is already on the higher side (around 8.3 or above), such as systems that already use kalkwasser.
Its gentle nature helps stabilize alkalinity without pushing pH beyond safe limits. Reefers who run open-top systems with strong gas exchange, or those living in areas with low ambient CO₂, often find bicarbonate dosing the safer option.
However, since bicarbonate solutions are less concentrated than carbonate ones, they require larger dosing containers or more frequent refills.
Sodium Carbonate: Stronger Alkalinity and a pH Boost
Sodium carbonate (Na₂CO₃), on the other hand, delivers a stronger alkalinity increase and raises pH. This makes it the go-to choice for reef tanks running at or below pH 8.2, where buffering capacity is lower and gas exchange might be limited.
Because sodium carbonate solutions are more concentrated, they dissolve efficiently and require smaller dosing containers.
For aquariums struggling with low pH, a combined approach works best:
- Dose sodium carbonate for alkalinity
- Add kalkwasser at night
- Improve ventilation
- Avoid tight-fitting lids that trap CO₂ and block light
Choosing the Right Tool for the Right Job
Ultimately, choosing between bicarbonate and carbonate depends on your tank’s pH profile:
- If pH > 8.3: Use sodium bicarbonate for stable alkalinity without further pH rise.
- If pH < 8.2: Use sodium carbonate to boost both alkalinity and pH.
Remember—what you dose doesn’t change the carbonate-to-bicarbonate ratio directly. Your pH, total alkalinity, temperature, and salinity determine that balance naturally.
Rather than chasing “perfect ocean ratios,” focus on keeping your alkalinity stable and your pH near natural seawater levels. Consistency, not marketing claims, leads to thriving corals and healthier reef ecosystems.

Final Thoughts on Alkalinity Management
Alkalinity is more than just a number—it’s a window into your reef’s chemical balance and coral growth potential. Whether you choose sodium bicarbonate or sodium carbonate, the key is understanding how each one interacts with your system’s pH.
Pick the right supplement for your tank’s needs, monitor alkalinity regularly, and make adjustments gradually. With careful attention, you’ll maintain stable conditions that let your corals grow strong, colorful, and resilient.
To purchase our pharmaceutical-grade sodium bicarbonate or sodium carbonate, click on the link below:
ACROKINGDOM ALK (alkalinity, sodium bicarbonate)- Pharmaceutical Grade 1Kg
ACROKINGDOM ALK (alkalinity, sodium carbonate)- Pharmaceutical Grade 600g
ACROKINGDOM Fundamental Care
Also check out our ACROPORA COLLECTION- CUT TO ORDER

